| Acids and Bases |
- Acid dissociation constant
- Acid-base extraction
- Acid–base reaction
- Acid–base titration
- Dissociation constant
- Acidity function
- Buffer solutions
- pH
- Proton affinity
- Self-ionization of water
- Acid strength
|
| Acid types |
- Brønsted
- Lewis
- Mineral
- Organic
- Strong
- Superacids
- Weak
|
| Base types |
- Brønsted
- Lewis
- Organic
- Strong
- Superbases
- Non-nucleophilic
- Weak
|
|
|
The strength of an acid refers to its ability or tendency to lose a proton. There are very few strong acids. A strong acid is one that completely ionizes (dissociates) in water; in other words, one mole of a strong acid HA dissolves in water yielding one mole of H+ and one mole of the conjugate base, A−. Essentially none of the non-ionized acid HA remains. In contrast a weak acid only partially dissociates, and at equilibrium both the acid and the conjugate base are present in solution. Examples of strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). In water each of these essentially ionizes 100%. The stronger an acid is, the more easily it loses a proton, H+. Two key factors that contribute to the ease of deprotonation are the polarity of the H—A bond and the size of atom A, which determines the strength of the H—A bond. Acid strengths are also often discussed in terms of the stability of the conjugate base.
Stronger acids have a larger Ka and a more negative pKa than weaker acids.
Sulfonic acids, which are organic oxyacids, are a class of strong acids. A common example is toluenesulfonic acid (tosylic acid). Unlike sulfuric acid itself, sulfonic acids can be solids. In fact, polystyrene functionalized into polystyrene sulfonate is a solid strongly acidic plastic that is filterable.
Superacids are acids stronger than 100% sulfuric acid. Examples of superacids are fluoroantimonic acid, magic acid and perchloric acid. Superacids can permanently protonate water to give ionic, crystalline hydronium "salts". They can also quantitatively stabilize carbocations.
|
Contents
- 1 Strong acids
- 1.1 Determining acid strength
- 1.2 Common strong acids
- 1.3 Almost strong acids
- 1.4 Extremely strong acids (as protonators)
- 2 Weak acids
- 2.1 Dissociation
- 2.2 Calculating the pH of a weak acid solution
- 2.2.1 Simplification
- 2.2.2 Comparison of the full and simplified methods
- 2.3 Conjugate acid/base pair
- 3 Factors determining acid strength
- 3.1 Polarity and the inductive effect
- 3.2 Atomic radius and bond strength
- 4 Corrosivity
- 5 References
- 6 External links
|
Strong acids
A strong acid is an acid that ionizes completely in an aqueous solution by losing one proton, according to the equation
- HA(aq) → H+(aq) + A−(aq)
For sulfuric acid which is diprotic, the "strong acid" designation refers only to dissociation of the first proton
- H2SO4(aq) → H+(aq) + HSO4−(aq)
More precisely, the acid must be stronger in aqueous solution than hydronium ion, so strong acids are acids with a pKa < −1.74. An example is HCl for which pKa = -6.3.[1] This generally means that in aqueous solution at standard temperature and pressure, the concentration of hydronium ions is equal to the concentration of strong acid introduced to the solution.
In all other acid-water reactions, dissociation is not complete, so will be represented as an equilibrium, not a completed reaction. The typical definition of a weak acid is any acid that does not dissociate completely. The difference separating the acid dissociation constants of strong acids from all other acids is so small that this is a reasonable demarcation.
Due to the complete dissociation of strong acids in aqueous solution, the concentration of hydronium ions in the water is equal to the total concentration (ionized and un-ionized) of the acid introduced to solution: [H+] = [A−] = [HA]total and pH = −log[H+].
Determining acid strength
The strength of an acid, in comparison to other acids, can be determined without the use of pH calculations by observing the following characteristics:
- Electronegativity: The higher the electronegativity of a conjugate base in the same period, the more acidic. In other words, the more electronegative A- is, more acidic (where HA -> H+ + A-).
- Atomic Radius: With increasing atomic radius, acidity also increases. For example, HCl and HI, both strong acids, ionize 100% in water to become their respective ionic constituents. However, HI is stronger than HCl. This is because the atomic radius of an atom of iodine is much larger than that of a chlorine atom. As a result, the negative charge over the I- anion is dispersed over a larger electron cloud and its attraction for the proton (H+) is not as strong as the same attraction in HCl. Therefore, HI is ionized (deprotonated) more readily.
- Charge: The more positively charged a species is, the more acidic (neutral molecules can be stripped of protons more easily than anions, and cations are more acidic than comparable molecules).
- Equilibrium: The strength of an acid can also be defined by the equilibrium position of its dissociation reaction:
HA(aq)+ H2O(l) → H3O+(aq) + A-(aq)
In a strong acid, equilibrium lies far to the right, meaning that almost all of the original HA is dissociated at equilibrium. A strong acid yields a weak conjugate base (A-), so a strong acid is also described as an acid whose conjugate base is a much weaker base than water.[2]
Common strong acids
This is a list of strong acids with pKa < -1.74, which is stronger than hydronium ion, from strongest to weakest.
- Hydroiodic acid HI (pKa = −9.3)[1]
- Hydrobromic acid HBr (pKa = −8.7)[1]
- Perchloric acid HClO4 (pKa ≈ −8)[3]
- Hydrochloric acid HCl (pKa = −6.3)[1]
- Sulfuric acid H2SO4 (first dissociation only, pKa1 ≈ −3)[3]
- p-Toluenesulfonic acid (pKa = −2.8) Organic soluble strong acid
Almost strong acids
These do not meet the strict criterion of being more acidic than H3O+, although in very dilute solution they dissociate almost completely, so sometimes they are included as "strong acids"
- Hydronium ion H3O+ (pKa = -1.74). Hydronium is often used as an approximation of the state of protons in water.
- Nitric acid HNO3 (pKa = -1.64)[3]
- Chloric acid HClO3 (pKa = -1.0)[3]
- Some chemists include bromic acid (HBrO3)[citation needed], perbromic acid (HBrO4)[citation needed], iodic acid (HIO3)[citation needed], and periodic acid (HIO4)[citation needed] as strong acids, although these are not universally accepted.
Extremely strong acids (as protonators)
(Strongest to weakest)
- Fluoroantimonic acid H[SbF6]
- Magic acid FSO3HSbF5
- Carborane superacid H(CHB11Cl11)
- Fluorosulfuric acid FSO3H
- Triflic acid CF3SO3H
Weak acids
Most acids are weak acids. A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution. These acids have higher pKa than strong acids, which release all of their hydrogen atoms when dissolved in water. Examples of weak acids include acetic acid (CH3COOH) and oxalic acid (H2C2O4).
Dissociation
Weak acids ionize in water solution to only a moderate extent; that is, if the acid was represented by the general formula HA, then in aqueous solution a significant amount of undissociated HA still remains. Weak acids in water dissociate as:
The strength of a weak acid is represented as either an equilibrium constant or as a percent dissociation. The equilibrium concentrations of reactants and products are related by the acid dissociation constant expression, (Ka):
The greater the value of Ka, the more the formation of H+ is favored, and the lower the pH of the solution. The Ka of weak acids varies between 1.8×10−16 and 55.5. Acids with a Ka less than 1.8×10−16 are weaker acids than water.
The other way to measure acid strength is to look at its percent dissociation, which is symbolized as α (alpha) and which can range from 0% < α < 100%. The percent dissociated is defined as
![\alpha = {{ \left[ A^- \right] } \over {\left[ A^- \right] + \left[ HA \right]}}](//upload.wikimedia.org/math/2/a/0/2a02eca08939cca0b8b70efd4af2627a.png)
Unlike Ka, α is not constant and does depend on the [HA]. In general α will increase as [HA] decreases. Thus acids become stronger as they are diluted. If acids are polyprotic, then each proton will have a Ka. For example: H2CO3 + H2O → HCO3– + H3O+ has two Ka values because it has two acidic protons. The first Ka value is 4.46×10−7 (pKa1 = 6.351) and the second is 4.69×10−11 (pKa2 = 10.329).
Calculating the pH of a weak acid solution
The pH of a solution of a weak acid depends on the strength of the acid and the other components in the solution. In the simplest case, the weak acid is the only compound in water. In this case, the pH can be found from the concentration of the acid (symbolized as ), from the of the acid (symbolized as HA), and by solving for concentration of H+ (symbolized by x and represented more accurately as H3O+). Below is a table that organizes the information. On the first line, the reaction is written. On the second line, the initial conditions are written below each compound. Note that a value of water is not given because its term (activity) in the expression is technically equal to 1, but is often (conveniently) omitted. The third line shows how the value changes as the reaction goes to equilibrium. Then the last line gives the equilibrium concentrations and is simply the sum of each column.
|
HA(aq) |
+ |
H2O(l) |
→ |
A–(aq) |
+ |
H3O+(aq) |
| initial |
F |
|
— |
|
0 |
|
0 |
| change |
-x |
|
— |
|
+x |
|
+x |
| equilibrium |
F - x |
|
— |
|
x |
|
x |
Applying the equilibrium line to the expression yields
![K_a = { {[H^+][A^-]} \over {[HA]} } = {{x^2} \over {F - x}}](//upload.wikimedia.org/math/3/8/8/388d5ea566217a7057d3b0ffee09e6ea.png)
rearranging yields , which can be solved for x using the quadratic equation. The pH is then calculated as .
Simplification
However, if F is more than 1000× greater than Ka, then (1) the acid will not deprotonate much, (2) the value of x will be small, and therefore (3) F - x ≈ F. This simplifies the Ka expression to...
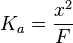
Solving for x yields
Then the pH = -log[H+]. The following equation then follows, but is only true if F >>> Ka

Comparison of the full and simplified methods
A certain weak acid has a Ka = 1×10−5 and the pH of two solutions needs to be found. One solution has a concentration of 0.10M and another has a concentration of 5×10−4M. The pH for both solutions will be calculated using both methods to yield 4 values, which will be compared.
0.1M Solution
The full method gives the following quadratic:
which gives x = 9.95×10−4 M and a pH = 3.00. The simplified method gives
So both methods yield the same result, but again F is more than 1000× greater than Ka. The next case does not have this condition and the results will differ.
5×10−4M Solution
The full method gives the following quadratic:
which gives x = 6.6×10−5 M and a pH = 4.18. The simplified method gives
Here, the results differ by 0.03 pH units. As F becomes closer in value to the Ka, then the difference will increase even more. However, in practice, it is rare to work with such dilute acids and the pH is also dependent on ionic strength and temperature. So in reality, the simplified method works well.
Conjugate acid/base pair
It is often stated that "the conjugate of a weak acid is a strong base". This statement can be misleading. Most weak acids that textbooks discuss have weak (not strong) conjugate bases. Truly, only the very weakest of acids have strong conjugate bases. For example, if a weak acid has a Ka = 10−5, then its conjugate base would have a Kb = 10−9 (from the relationship Ka × Kb = 10−14), which certainly is not a strong base. A very weak acid with a Ka = 10−20 would indeed have a strong conjugate base.
Factors determining acid strength
Polarity and the inductive effect
Polarity refers to the distribution of electrons in a bond, the region of space between two atomic nuclei where a pair of electrons is shared. When two atoms have roughly the same electronegativity (ability to attract electrons) the electrons are shared evenly and spend equal time on either end of the bond. When there is a significant difference in electronegativities of two bonded atoms, the electrons spend more time near the nucleus of the more electronegative element and an electrical dipole, or separation of charges, occurs, such that there is a partial negative charge localized on the electronegative element and a partial positive charge on the electropositive element. Hydrogen is an electropositive element and accumulates a slightly positive charge when it is bonded to an electronegative element such as oxygen or bromine. As the electron density on hydrogen decreases it is more easily abstracted, in other words, it is more acidic. Moving from left to right across a row on the periodic table elements become more electronegative (excluding the noble gases), and the strength of the binary acid formed by the element increases accordingly:
| Formula |
Name |
pKa[4] |
| HF |
hydrofluoric acid |
3.17 |
| H2O |
water |
15.7 |
| NH3 |
ammonia |
38 |
| CH4 |
methane |
48 |
The electronegative element need not be directly bonded to the acidic hydrogen to increase its acidity. An electronegative atom can pull electron density out of an acidic bond through the inductive effect. The electron-withdrawing ability diminishes quickly as the electronegative atom moves away from the acidic bond. The effect is illustrated by the following series of halogenated butanoic acids. Chlorine is more electronegative than bromine and therefore has a stronger effect. The hydrogen atom bonded to the oxygen is the acidic hydrogen. Butanoic acid is a carboxylic acid.
| Structure |
Name |
pKa[5] |
|
butanoic acid or butyric acid |
≈4.8 |
|
4-chlorobutanoic acid |
4.5 |
|
3-chlorobutanoic acid |
≈4.0 |
|
2-bromobutanoic acid |
2.93 |
|
2-chlorobutanoic acid |
2.86 |
As the chlorine atom moves further away from the acidic O—H bond, its effect diminishes. When the chlorine atom is just one carbon removed from the carboxylic acid group the acidity of the compound increases significantly, compared to butanoic acid (a.k.a. butyric acid). However, when the chlorine atom is separated by several bonds the effect is much smaller. Bromine is much more electronegative than either carbon or hydrogen, but not as electronegative as chlorine, so the pKa of 2-bromobutanoic acid is slightly greater than the pKa of 2-chlorobutanoic acid.
Perchloric acid (HClO
4) is an oxoacid and a strong acid.
The number of electronegative atoms adjacent an acidic bond also affects acid strength. Oxoacids have the general formula HOX where X can be any atom and may or may not share bonds to other atoms. Increasing the number of electronegative atoms or groups on atom X decreases the electron density in the acidic bond, making the loss of the proton easier. Perchloric acid is a very strong acid (pKa ≈ -8) and completely dissociates in water. Its chemical formula is HClO4 and it comprises a central chlorine atom with three chlorine-oxygen double bonds (Cl=O) and one chlorine-oxygen single bond (Cl—O). The singly bonded oxygen bears an extremely acidic hydrogen atom which is easily abstracted. In contrast, chloric acid (HClO3) is a weaker acid, though still quite strong (pKa = -1.0), while chlorous acid (HClO2, pKa = +2.0) and hypochlorous acid (HClO, pKa = +7.53) acids are weak acids.[6]
Carboxylic acids are organic acids that contain an acidic hydroxyl group and a carbonyl (C=O bond). Carboxylic acids can be reduced to the corresponding alcohol; the replacement of an electronegative oxygen atom with two electropositive hydrogens yields a product which is essentially non-acidic. The reduction of acetic acid to ethanol using LiAlH4 (lithium aluminium hydride or LAH) and ether is an example of such a reaction.
The pKa for ethanol is 16, compared to 4.76 for acetic acid.[5][7]
Atomic radius and bond strength
Another factor that contributes to the ability of an acid to lose a proton is the strength of the bond between the acidic hydrogen and the atom that bears it. This, in turn, is dependent on the size of the atoms sharing the bond. For an acid HA, as the size of atom A increases, the strength of the bond decreases, meaning that it is more easily broken, and the strength of the acid increases. Bond strength is a measure of how much energy it takes to break a bond. In other words, it takes less energy to break the bond as atom A grows larger, and the proton is more easily removed by a base. This partially explains why hydrofluoric acid is considered a weak acid while the other hydrohalic acids (HCl, HBr, HI) are strong acids. Although fluorine is more electronegative than the other halogens, its atomic radius is also much smaller, so it shares a stronger bond with hydrogen. Moving down a column on the periodic table atoms become less electronegative but also significantly larger, and the size of the atom tends to dominate its acidity when sharing a bond to hydrogen. Hydrogen sulfide, H2S, is a stronger acid than water, even though oxygen is more electronegative than sulfur. Just as with the halogens, this is because sulfur is larger than oxygen and the H—S bond is more easily broken than the H—O bond.
Corrosivity
While strong acids are generally assumed to be the most corrosive, this is not always true. The carborane superacid H(CHB11Cl11), which is one million times stronger than sulfuric acid,[8][9] is entirely non-corrosive, whereas the weak acid hydrofluoric acid (HF) is corrosive and can dissolve, among other things, glass[10] and most metals.
References
- Hill, John W., et al. "General Chemistry." 4th ed. New Jersey: Prentice Hall, 2005.
- ^ a b c d William L. Jolly "Modern Inorganic Chemistry" (McGraw-Hill, 1984), p.177
- ^ Zumdahl, Steven S. (2011). Chemical Principles: Enhanced Edition, Sixth Editiion. Brooks/Cole Cengage Learning. p. 236.
- ^ a b c d Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 171. ISBN 978-0130399137.
- ^ pKa's of Inorganic and Oxo-Acids
- ^ a b Section 8: Electrolytes, Electromotive forces and Chemical Equilibrium
- ^ pKa values for HClOn from Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0130399137.
- ^ pKa Data Compiled by R. Williams
- ^ George A. Olah, et. al. Superacid Chemistry, 2nd ed., Wiley, p. 41.
- ^ That is, the ability of the carborane superacid to protonate a given base (B) is one million times greater than a solution of sulfuric acid, so that the ratio [BH+] / [B] is one million times higher. The relative acidities of strong acids can be evaluated using the Hammett acidity function.
- ^ CID 14917 from PubChem
External links
- http://www.cm.utexas.edu/academic/courses/Spring2002/CH301/McDevitt/strong.htm
- http://jchemed.chem.wisc.edu/Journal/Issues/2000/Jul/abs849.html
- Titration of acids - freeware for data analysis and simulation of potentiometric titration curves
- Acids and Bases - definitions
![\alpha = {{ \left[ A^- \right] } \over {\left[ A^- \right] + \left[ HA \right]}}](http://upload.wikimedia.org/math/2/a/0/2a02eca08939cca0b8b70efd4af2627a.png)
![K_a = { {[H^+][A^-]} \over {[HA]} } = {{x^2} \over {F - x}}](http://upload.wikimedia.org/math/3/8/8/388d5ea566217a7057d3b0ffee09e6ea.png)